We use cookies to operate this website and to improve its usability. Full details of what cookies are, why we use them and how you can manage them can be found by reading our Privacy & Cookies page. Please note that by using this site you are consenting to the use of cookies.
Pharmaceuticals
More safety and convenience for pharmaceutical packaging
2018-04-08
The medical sector, in particular, is an area where everything has to be done rather quickly at times, often using only one hand. To cater for this need, Schreiner Medipharm has developed a new labelling solution for syringes. With Pharma-Multi-Act the label is quite simply opened with a single hand movement while opening the cap.
GUARANTEED INITIAL OPENING INDICATION
To ensure single use and to eliminate potential manipulation, opening of the cap automatically involves tearing along a perforation on the tab without affecting the injection process in any way.
As well as providing initial opening protection, the label has a range of further indicator functions. To make an entry in a patient file or vaccination certificate, a documentary tab can be detached from the cap, even while wearing gloves. The area below can be used as an indicator field for a variety of purposes, such as hidden warnings, safety features for verification, NFC chips as well as temperature and UV indicators.
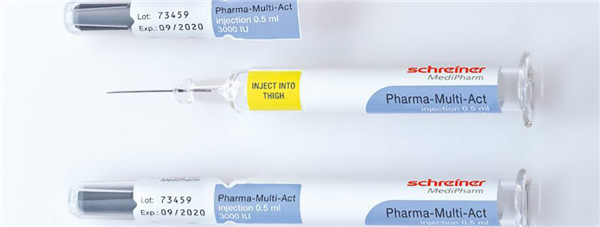 The new labelling solution for syringes from Schreiner MediPharm is flexible to handle, while also offering counterfeit protection. Photo: Schreiner MediPharm
The new labelling solution for syringes from Schreiner MediPharm is flexible to handle, while also offering counterfeit protection. Photo: Schreiner MediPharm
DIGITAL PRINTING FOR SMALL BATCH SIZES
Another special feature of the pharmaceutical sector is the need for individually prepared drugs that often have to be manufactured and packaged in small batches. For Iscador, a company specialising in anthroposophical mistletoe preparations, this is in fact indispensable. For tumour and cancer patients medication is always made in small batches of 80 to 2,000 packages. Moreover, the packages then need to be sent to numerous different countries, resulting in a large number of packages. To save storage expenses, the pharmaceutical company has decided to produce all its products in-house.
A suitable packaging solution, complete with logistics, is provided by the specialist packaging company Atlantic Zeiser. Their new printing and software solution MEDTRACKER makes it possible to print the required country markings on the packaging quickly, flexibly and without any time-consuming hardware conversion. In addition, the software allows the user to generate, print, control and manage serialised codes. Until recently, such codes had to be applied at the plant, using a cartridge printer, which resulted in up to 30 per cent rejects and quite often machine stoppages.
.jpg) MEDTRACKER is a modular software solution that is specially tailored to suit serialisation and track & trace applications in the pharmaceutical sector. Photo: Atlantic Zeiser
MEDTRACKER is a modular software solution that is specially tailored to suit serialisation and track & trace applications in the pharmaceutical sector. Photo: Atlantic Zeiser
COOL ENVIRONMENT
The Swiss company also has to provide a cool environment as a special condition for the packaging process. Ampoules with liquid medication need to be kept at a consistent temperature between two and eight degrees Celsius, and the packaging process is no exception. Until the new software was introduced, the only option was to apply the dual verification principle. Thanks to the new data management system, compliance with the required chilling consistency can now be communicated to wholesale customers in a way that is more reliable and time-efficient.
GUARANTEED INITIAL OPENING INDICATION
To ensure single use and to eliminate potential manipulation, opening of the cap automatically involves tearing along a perforation on the tab without affecting the injection process in any way.
As well as providing initial opening protection, the label has a range of further indicator functions. To make an entry in a patient file or vaccination certificate, a documentary tab can be detached from the cap, even while wearing gloves. The area below can be used as an indicator field for a variety of purposes, such as hidden warnings, safety features for verification, NFC chips as well as temperature and UV indicators.

DIGITAL PRINTING FOR SMALL BATCH SIZES
Another special feature of the pharmaceutical sector is the need for individually prepared drugs that often have to be manufactured and packaged in small batches. For Iscador, a company specialising in anthroposophical mistletoe preparations, this is in fact indispensable. For tumour and cancer patients medication is always made in small batches of 80 to 2,000 packages. Moreover, the packages then need to be sent to numerous different countries, resulting in a large number of packages. To save storage expenses, the pharmaceutical company has decided to produce all its products in-house.
A suitable packaging solution, complete with logistics, is provided by the specialist packaging company Atlantic Zeiser. Their new printing and software solution MEDTRACKER makes it possible to print the required country markings on the packaging quickly, flexibly and without any time-consuming hardware conversion. In addition, the software allows the user to generate, print, control and manage serialised codes. Until recently, such codes had to be applied at the plant, using a cartridge printer, which resulted in up to 30 per cent rejects and quite often machine stoppages.
COOL ENVIRONMENT
The Swiss company also has to provide a cool environment as a special condition for the packaging process. Ampoules with liquid medication need to be kept at a consistent temperature between two and eight degrees Celsius, and the packaging process is no exception. Until the new software was introduced, the only option was to apply the dual verification principle. Thanks to the new data management system, compliance with the required chilling consistency can now be communicated to wholesale customers in a way that is more reliable and time-efficient.






